Reducing human errors throughout the lab is a major concern for life science companies and in recent years many resources are directed to developing internal practices and processes to deal with human errors reduction. Human error reduction (HER) can lead to reducing operational and drug development costs, shorten drug development cycles, and foster higher product quality, not to mention preventing accidents. Human errors are responsible for quite a large number of FDA warning letters and it is a high priority for labs to ensure data integrity and compliance with laws and regulations.
There are quite a few precautions that can be taken to prevent human errors in the lab. Using an Electronic Lab Notebook (ELN) or informatics platform will enable you to automate data-driven processes, properly track procedures and perform some tasks automatically rather than manually. Following are some examples that demonstrate how using an ELN can prevent and reduce human errors:
Structuring work processes
Structuring data in controlled and pre-defined format enables to collect data and analyze it in a methodical form. Predefining the way results are being documented by creating a structured form makes it easy to supervise the ongoing work in the lab. Forms minimize errors by defining limitations in advance, such as offering limited options to choose from on certain fields, thus forcing lab members to follow a predefined format, minimizing the option to enter data freely.
Unstructured data is divided into various formats such as spreadsheets, emails, word files etc. Working with unstructured data exposes you to human errors- may it be manual data entries, unclear work procedures and ambiguous experimental steps. The ability to structure unstructured scientific data and create enforced unified formats to collect data can not only reduce human errors but also be more productive and cost-effective.
Workflows
As the industry is becoming more data-driven, big data has become a major challenge. Deriving insights from data has become more difficult, time-consuming, and susceptive to human errors. In addition, the need to automate and streamline lab processes is a key factor in enhancing lab operations. Customizing and automating a series of actions and events that occur in the lab and creating automated workflows can reduce human errors considerably. In addition processes automation also helps reduce human errors that occur when processes are done manually.
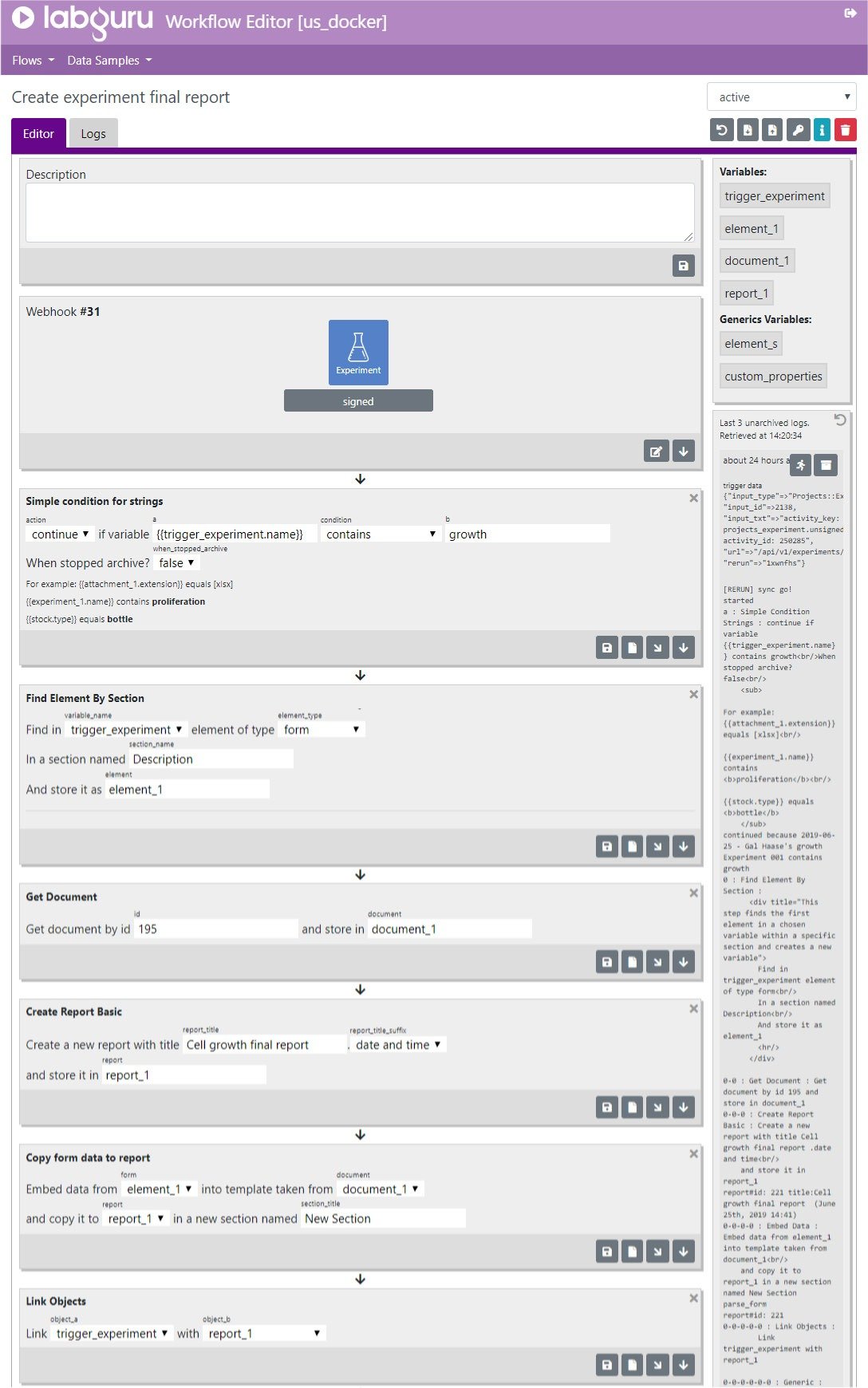
Labguru Workflow Editor
Calibration
Searching through FDA warning letters issued in the past few years with regards to calibration demonstrates that failure to calibrate equipment on time and document calibration procedures are recurring issues that surface time after time. It is easy to avoid such failures and maintain data integrity and traceability simply by using a calibration management software that will enable to track equipment maintenance on a regular basis. Using an ELN that includes a calibration module will enable you to manage all your calibration tasks in one centralized platform.
Labelling
Barcode labelling improves data collection and management, helps streamline inventory management and sharply decreases human errors.
Labs that rely on manual data entries are exposed to human errors such as wrong entry of numbers, poor handwriting, missing a line, or omitting a number. In addition, time is also wasted on finding the errors and fixing them. Using barcodes almost eliminates human errors in sample labelling, saves time searching as scanning barcodes are fast and reliable and result in fast and reliable answers.
Using an ELN with a labelling system will allow you to easily track your samples, tubes and boxes by printing labels directly from your electronic lab book and later quickly retrieve the sample information and keep track of your inventory within the ELN.
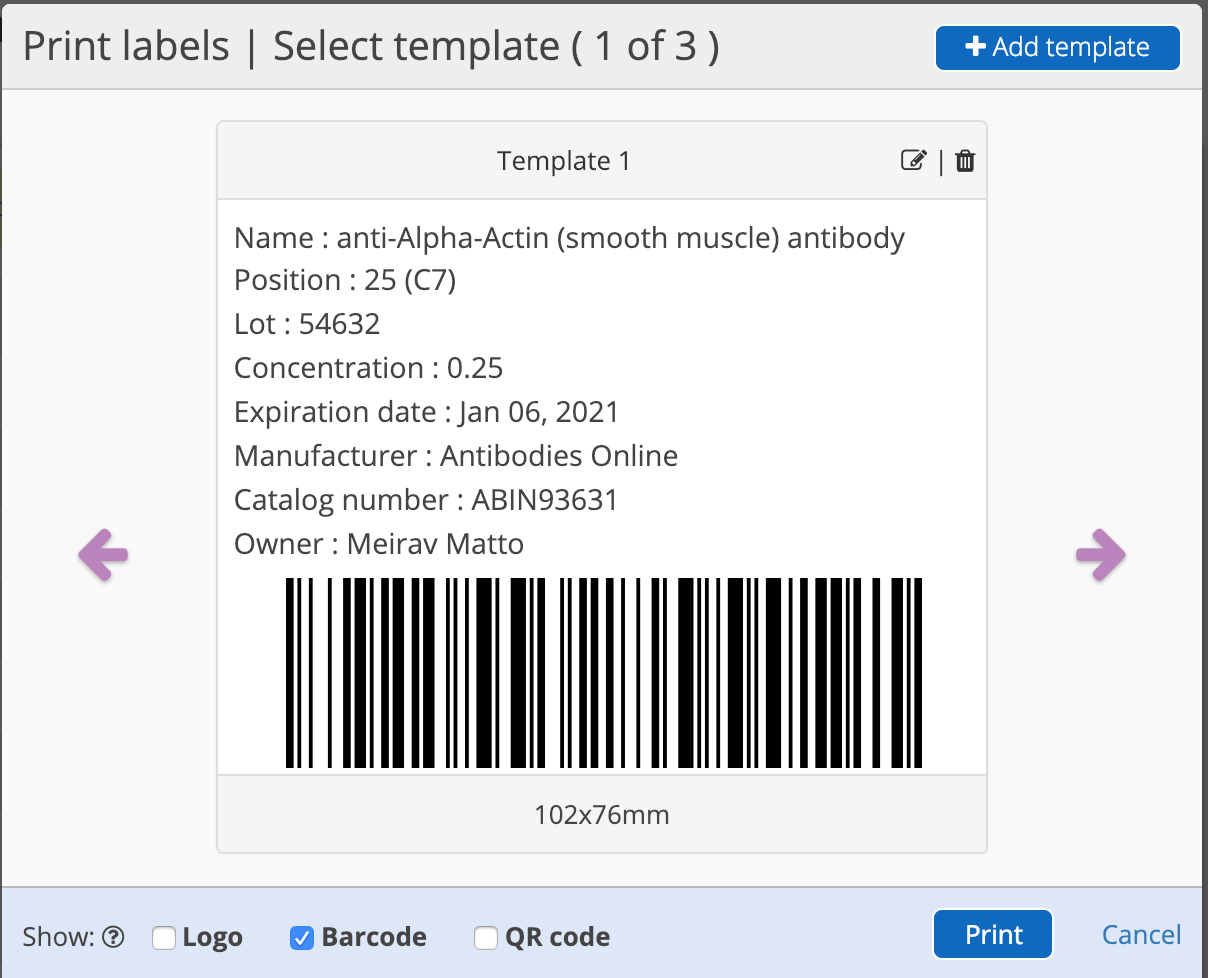
Inventory Management
Keeping track of orders manually is a sure way to be susceptible to human errors. It is most annoying to find out that a certain material is out of stock or not to be found when you are about to perform an experiment. Using a lab inventory management system as part of your ELN will enable you to track your orders systematically and efficiently, eliminate duplicate orders, avoid misplacement of materials or improper cataloguing.
Automating processes and leaning on a laboratory management system to mitigate the risk of human errors will enable your organization to focus on development, free time of your lab members and better comply with FDA regulations.
To learn more about how Labguru ELN can help you reduce human errors- book a demo.


%20(4).png)