Centralize and share information easily with customers and regulatory inspectors
Contract Research Organizations (CROs) and Contract Development and Manufacturing Organizations (CDMOs) need to meet strict standards and present a detailed and accurate report of all actions performed in the course of their clinical trials — both for customers and regulatory inspections.
Labguru is a cloud-based ELN, informatics, and lab management platform that allows CROs and CDMOs to effortlessly centralize information from various sources and formats, structure and organize it, share it, and create reports.
Centralize Information
-
Organize your information and create different projects for different customers
-
Manage all protocols and SOPs in one place
-
Share documents and experiments across departments and teams
-
Structure your notes using reusable form templates
-
Track every action with time-stamped experiment steps
Share Information
-
Grant customers viewing permissions so they can see updates in real-time
-
Create and visualize reports in tables and graphs to track and share specific details
-
Use workflows to automate the creation of submission-ready documents and reports
Ensure Data Integrity
-
A full audit trail: every action in the system is recorded and available for viewing with details including user, date and time, and action.
-
Use time-stamped digital signatures (sign-and-witness) to lock experiments to further changes
-
Access all historical versions of entities in the system
Lab Management Software That Gets Results
Save time, reduce lab costs and prevent human error
The Labguru lab management software optimizes clinical operations by providing tools for inventory and storage management, equipment calibration and maintenance, sample management, and more. Labguru empowers CROs to stay on top of work performed across multiple departments and manage many projects simultaneously with minimum expenses and maximum efficiency.
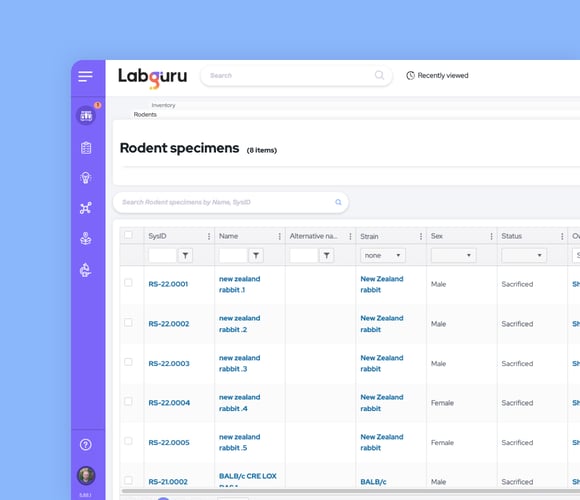
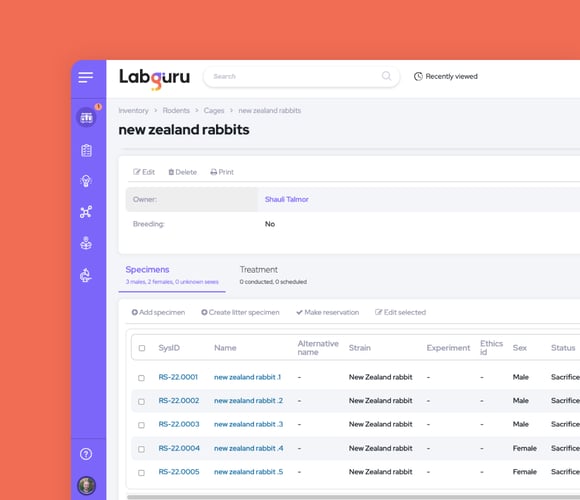
Optimize Animal Colony Management
-
Use specialized animal inventory collections to document every strain and specimen
-
Track lineages and parent-child connections
-
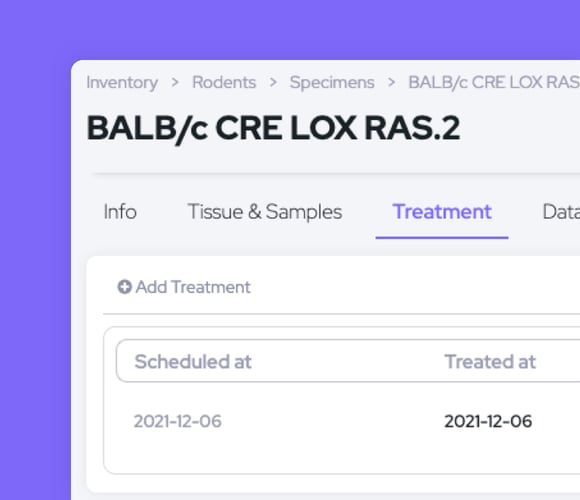
Track treatments for each specimen and create reports with details such as weight, treatment type/quantity/time, and clinical data.
-
Customize collections to meet your needs
Save time & Increase Efficiency
- Link stocks to physical lab locations so you can always find what you need
- Manage equipment calibration and maintenance and schedule equipment sessions
- Design and print QR-coded or barcode labels and use them to scan item information directly to the database.
- Link samples and supplies to experiment pages
- Automate data management processes using the Workflow Editor
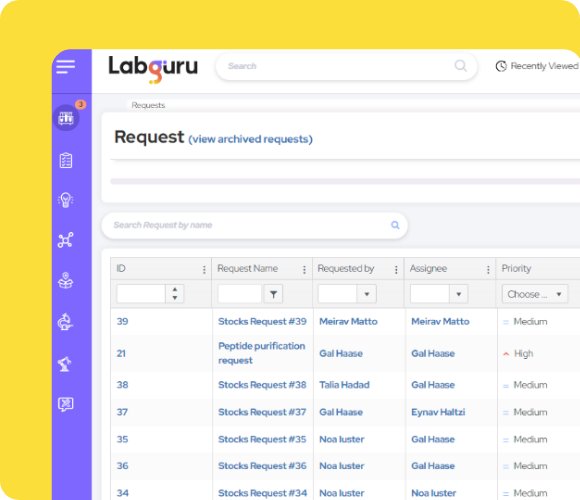
New! Requests Module: Streamlining CRO Operations for Success
Optimize your operations utilizing our centralized Request Module to streamline request handling and enhance project success, ensuring a new level of organization, transparency, and efficiency:
- Customizable Templates for Project Specificity: Create custom request templates tailored to each project's unique requirements, ensuring clarity and precision in CRO operations.
- Efficient Resource Allocation: Prioritize and manage sample testing and shipping requests efficiently, allowing your team to allocate resources effectively and meet project deadlines.
- Seamless Collaboration: Foster clear communication and teamwork with real-time updates on request status and assignments, enabling effective project management.
- Integrated Data Management: The Requests module seamlessly integrates with ELN, LIMS, inventory, and equipment modules, providing familiar tools for streamlined data management.
Why Labguru?
We are an all-in-one solution
Conduct efficient clinical trials and maximize your research output. Plan and document trials, track progress, streamline logistics, and share results.
We support you from planning to reporting
Streamline everything from clinical trial planning to compliant reporting on one centralized platform employing advanced technological tools.
We're here to meet your needs
We'd love to learn more about your unique needs to create a tailored solution for your trials. Just let us know how we can streamline your research.
Our solution is intuitive and easy to use
Forget about lengthy onboarding processes and training sessions. Labguru is designed to be so intuitive, you can pick up where you left off.
Labguru’s holistic ELN, LIMS, and informatics solution was created by scientists for scientists
Our customer success team of science PhDs will gladly assist you in implementation, customization of the system to your needs, day-to-day operations, and more.